KAWANO Jun
Associate Professor
Understanding atomic-scale mechanisms of mineral growth
Department of Earth and Planetary Sciences, Earth and Planetary System Science

| Theme | Experimental and theoretical investigation for metastable formation mechanism of carbonate minerals |
| Field | Mineralogy, Crystal Growth |
| Keyword | Minerals, Crystal Growth, Biomineralization, Mineral/water interface, Metastable phase, Amorphous, Carbonate minerals, Molecular simulation |
Introduction of Research
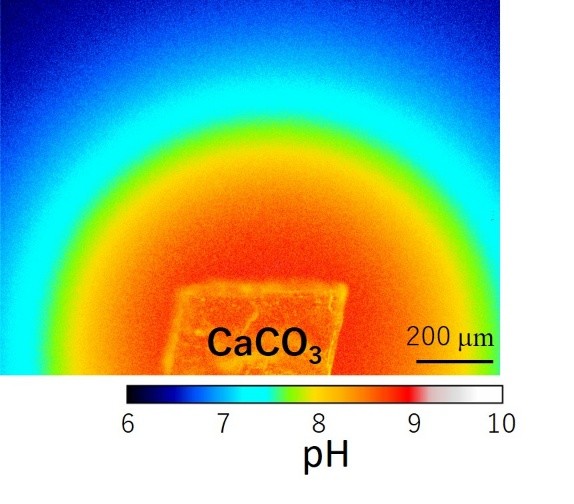
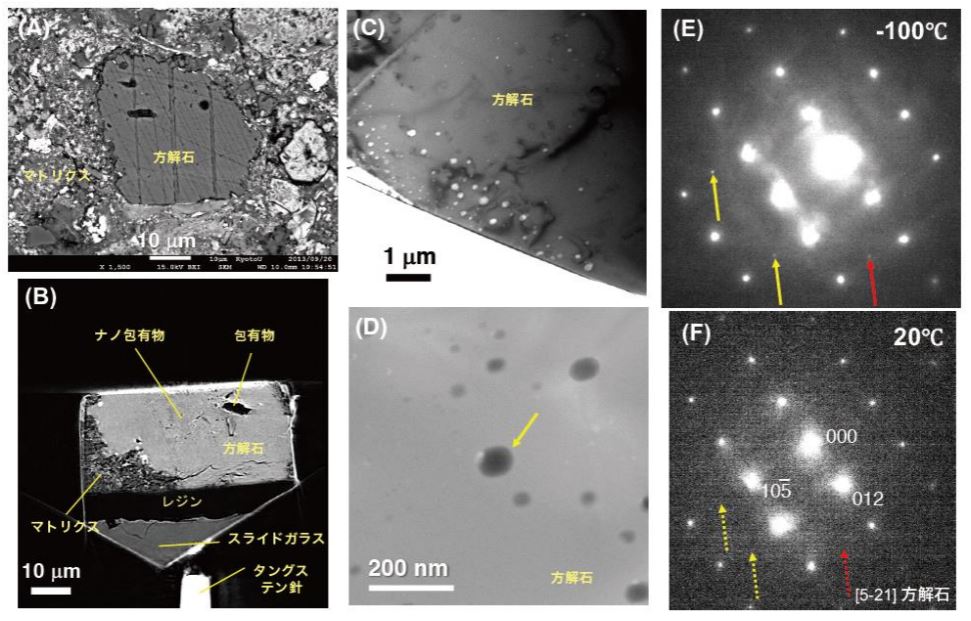
My research interests are in understanding crystal growth and phase transformation mechanisms of various minerals based on both experimental and computational techniques. Especially, I would like to know the nucleation and surface process of carbonate minerals, which are common biominerals forming biological hard tissues like shells and coral skeletons.
Representative Achievements
Related industries
| Academic degree | Ph.D. |
| Academic background | 1998 B.Sci. Faculty of Science, Kyoto University 2000 M.Sci. Graduate School of Science, Kyoto University 2003 Ph.D. Graduate School of Science, Kyoto University 2003-2005 Postdoctoral Fellow, Kyoto University 2005-2007 Lecturer, Yamanashi Institute of Gemology and Jewelry Art 2007-2010 Researcher, Research Laboratory, Gemological Association of All Japan 2010-2011 Research Associate, Research Institute of Applied Mechanics, Kyusyu University 2011 Research Assistant Professor, Research Institute of Applied Mechanics, Kyusyu University 2011-2016 Assistant Professor, Creative Research Institution, Hokkaido University 2016-present |
| Affiliated academic society | Japan Association of Mineralogical Sciences, The Japanese Association of Crystal Growth, The Molecular Simulation Society of Japan, Mineralogical Society of America, The Japan Society of Applied Physics |
| Room address | Faculty of Science Building 6 2006-08-04 |